Since July 15, 2024, Côte d’Ivoire has introduced the R21/Matrix-M malaria into the vaccination schedule for children aged 6 to 23 months. This new vaccine is being gradually rolled out across the country as part of the Expanded Program on Immunization (EPI). The Ivorian Ministry of Health has already received 656,600 doses of the vaccine, with 131,100 doses delivered on June 27, 2024.
The government aims to vaccinate 250,000 children in 38 health districts across 16 regions before extending it to the remaining 75 health districts by the end of the year. This initiative is part of the fight against malaria, a disease that remains the leading cause of medical consultations in Côte d’Ivoire despite a decrease in malaria-related deaths from 3,222 in 2017 to 1,316 in 2020.
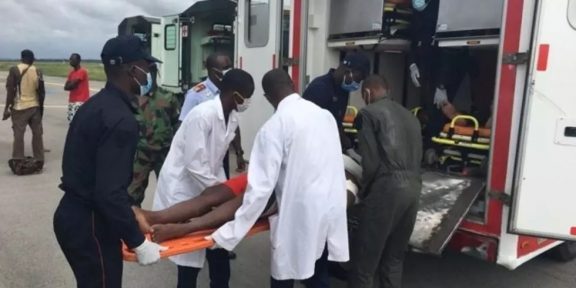
However, concerns are growing about the safety of this vaccine. Information circulating on social media recently reported the death of a little girl after receiving a dose of the vaccine in the Abobo district, northeast of Abidjan. This incident has alarmed parents and healthcare professionals about the potential side effects of R21/Matrix-M.
The World Health Organization (WHO) has nonetheless given its approval for the use of R21/Matrix-M, stating that is safe and about 77% effective. Nevertheless, doubts persist. The R21/Matrix-M is the second malaria vaccine recommended by the WHO, after the RTS,S/AS01. It was created to reduce child mortality due to malaria, which caused more than 600,000 deaths worldwide in 2022, with 95% in Africa and 80% among children under five.
The Ministry of Health, under the direction of Mr. Pierre N’gou Dimba, strongly supports the introduction of the vaccine, emphasizing the need to protect children against malaria. They plan to administer the vaccine in four doses at 6, 8, 9, and 15 months. Dr. Aka Charles Koffi, Chief of Staff at the Ministry of Health, emphasized that this vaccination campaign is crucial for reducing child mortality rates and combating vector resistance to insecticides.
Despite these assurances, the introduction of this vaccine has sparked controversy. Parents are encouraged to vaccinate their children, but some remain skeptical of the government’s enthusiasm. The possible side effects of R21/Matrix-M and the recent reported incidents raise questions about the haste of this vaccination campaign.
The main criticism is that Côte d’Ivoire has allowed the use of this vaccine, which has not been sufficiently tested, on its children. It is unacceptable that unproven vaccines are deployed in Africa, turning African children into guinea pigs for Western clinical trials. Ivorian authorities must take responsibility for this hasty and potentially dangerous decision.
The deployment of R21/Matrix-M in Côte d’Ivoire is part of a broader effort to eliminate malaria in Africa. Countries such as Ghana, Nigeria, Burkina Faso, and the Central African Republic have already approved this vaccine. However, the controversy surrounding its safety could slow its acceptance and effectiveness in the fight against malaria. Ivorian authorities must reconsider their position and prioritize the safety and well-being of their children before yielding to international pressures.